Autoclave Machine Risk Assessment . This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical. Control of automated and manual systems; It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. An autoclave is capable of rendering items sterile of any living organisms by. 11 you must assess the following key issues: At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of.
from www.segurohealthandsafety.co.uk
It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. 11 you must assess the following key issues: At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of. General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. Control of automated and manual systems; An autoclave is capable of rendering items sterile of any living organisms by. This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical.
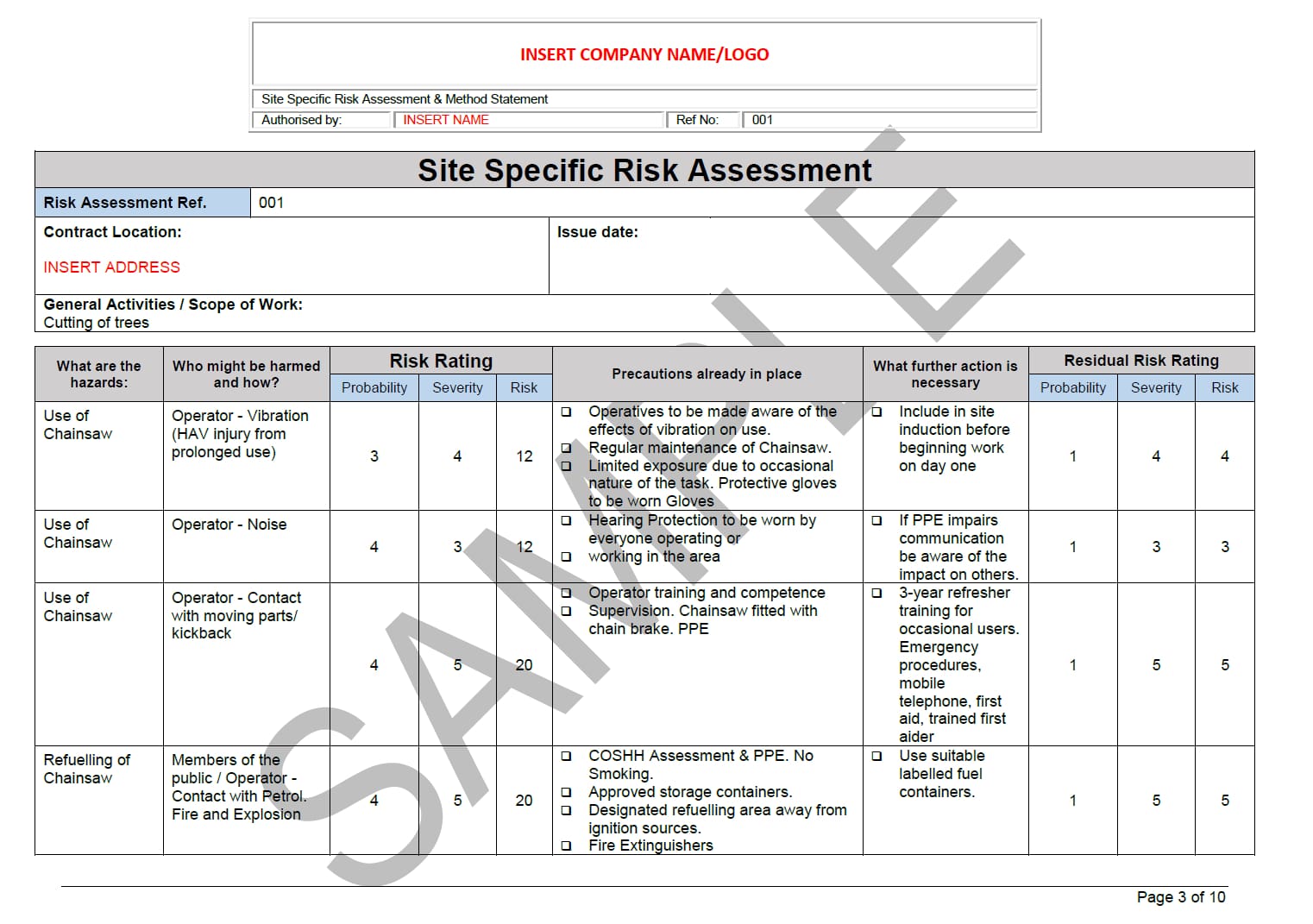
Risk & Method Statement for Tree Work Seguro
Autoclave Machine Risk Assessment This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical. An autoclave is capable of rendering items sterile of any living organisms by. 11 you must assess the following key issues: At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of. This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical. General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. Control of automated and manual systems; It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough.
From chemistnotes.com
Autoclave Machine Definition, Reliable function, and 2 Major Autoclave Machine Risk Assessment An autoclave is capable of rendering items sterile of any living organisms by. This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical. It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. General risk assessment a suitable and sufficient risk assessment. Autoclave Machine Risk Assessment.
From www.rradar.com
Autoclave and Industrial Oven Risk Assessment Autoclave Machine Risk Assessment General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. Control of automated and manual systems; At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of. This assessment covers the use of laboratory. Autoclave Machine Risk Assessment.
From www.dreamstime.com
Steam Sterilization of Equipment Used in SPA Procedures by Autoclave Autoclave Machine Risk Assessment 11 you must assess the following key issues: Control of automated and manual systems; This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical. It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. An autoclave is capable of rendering items sterile. Autoclave Machine Risk Assessment.
From www.researchgate.net
(PDF) Aseptic Transfer Risk Assessment A Case Study Autoclave Machine Risk Assessment It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid. Autoclave Machine Risk Assessment.
From www.scribd.com
Autoclaves Generic Assessment l Use of Autoclaves Risk Safety Autoclave Machine Risk Assessment General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. Control of automated and manual systems; At the very basic stage of design the risk assessment is to verify that all features. Autoclave Machine Risk Assessment.
From dokumen.tips
(PDF) Risk Assessment Autoclavesmedicine.nus.edu.sg/gsl/safety/risk Autoclave Machine Risk Assessment Control of automated and manual systems; It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. An autoclave is capable of rendering items sterile of any living organisms by. General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. 11 you must. Autoclave Machine Risk Assessment.
From kalstein.com.mx
La importancia del autoclave en un hospital Kalstein Autoclave Machine Risk Assessment It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. 11 you must assess the following key issues: This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical. At the very basic stage of design the risk assessment is to verify that. Autoclave Machine Risk Assessment.
From mavink.com
Task Risk Assessment Template Autoclave Machine Risk Assessment At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of. 11 you must assess the following key issues: Control of automated and manual systems; An autoclave is capable of rendering items sterile of any living organisms by. It can also help notified bodies. Autoclave Machine Risk Assessment.
From www.priorclave.com
9 Questions to Help You Find the Right Autoclave Autoclave Machine Risk Assessment 11 you must assess the following key issues: At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of. Control of automated and manual systems; It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. This. Autoclave Machine Risk Assessment.
From www.machinesafetyspecialists.com
ANSI/RIA Compliant Risk Assessment Spreadsheet Machine Safety Specialists Autoclave Machine Risk Assessment This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical. General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. At the very basic stage of design the risk assessment is to verify that all features are taken into consideration. Autoclave Machine Risk Assessment.
From lerablog.org
What You Need to Know About Autoclave Sterilization Autoclave Machine Risk Assessment Control of automated and manual systems; 11 you must assess the following key issues: It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. This assessment covers the use of laboratory autoclaves. Autoclave Machine Risk Assessment.
From worksafetyqld.com
Free Risk Assessment Tool Work Safety QLD Autoclave Machine Risk Assessment At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of. Control of automated and manual systems; It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. An autoclave is capable of rendering items sterile of. Autoclave Machine Risk Assessment.
From www.aiophotoz.com
Machinery Risk Assessment Template Sampletemplatess Sampletemplatess Autoclave Machine Risk Assessment This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical. 11 you must assess the following key issues: At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of. It can also help notified. Autoclave Machine Risk Assessment.
From slideplayer.com
General Laboratory Equipment ppt download Autoclave Machine Risk Assessment At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of. An autoclave is capable of rendering items sterile of any living organisms by. It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. This assessment. Autoclave Machine Risk Assessment.
From www.dexform.com
Autoclave log sample in Word and Pdf formats Autoclave Machine Risk Assessment Control of automated and manual systems; This assessment covers the use of laboratory autoclaves for sterilising infected materials other than hazard group 4 biological agents, medical. General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. It can also help notified bodies (responsible for conformity assessment) and competent persons (devising. Autoclave Machine Risk Assessment.
From slideplayer.com
AUTOCLAVES to an online ppt download Autoclave Machine Risk Assessment It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. At the very basic stage of design the risk assessment is to verify that all features are taken into consideration to avoid the risk of failure of. An autoclave is capable of rendering items sterile of any living organisms by. General risk. Autoclave Machine Risk Assessment.
From safety.umbc.edu
Autoclaves & Autoclave Waste Disposal Environmental Safety and Health Autoclave Machine Risk Assessment An autoclave is capable of rendering items sterile of any living organisms by. General risk assessment a suitable and sufficient risk assessment must be completed by a competent person before using an autoclave. It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. This assessment covers the use of laboratory autoclaves for. Autoclave Machine Risk Assessment.
From www.microlit.com
What is Autoclaving?, Sterilization Method for Laboratory Instruments Autoclave Machine Risk Assessment 11 you must assess the following key issues: An autoclave is capable of rendering items sterile of any living organisms by. It can also help notified bodies (responsible for conformity assessment) and competent persons (devising written schemes of thorough. Control of automated and manual systems; At the very basic stage of design the risk assessment is to verify that all. Autoclave Machine Risk Assessment.